Why purify the air of a refuge chamber?
The air in an emergency shelter is at risk of being polluted by human presence: breathing or outside pollution (misuse of the airlock). The danger of such pollution in an enclosed space is the increase in concentrations of Carbon Monoxide (CO) and Carbon Dioxide (CO2) which can reach lethal thresholds.
For a refuge not supplied with fresh air, it is therefore recommended to use a scrubber: a machine used to purify the gases. This scrubber generally uses interchangeable filter cartridges.
SUB’ROCA offers a turnkey solution to ensure real-time security of the refuge’s atmosphere thanks to its SENSOR’BOX. This multi-gas beacon is designed to be simple and intuitive to use. It monitors the air quality of your underground work environment at all times and in all places.

Carbon Dioxide – CO2
Carbon dioxide, an odourless and colourless gas, is present in the atmosphere at a level of 413 ppmv (parts per million by volume) or 0.0413%. CO2 is emitted by the respiration of living beings and the combustion of fossil fuels such as oil, coal or natural gas.
A concentration of 20% CO2 can lead to asphyxiation and death.
The level of CO2 intoxication varies according to the concentration and duration of exposure.
A concentration of 5,000 ppm, i.e. a value of 0.5%, for regular exposure over eight working hours is generally accepted by the various competent international bodies:
- The eight-hour weighted average value for the European Union (Directive 91/322/EC);
- The TLV-TWA (time-weighted average) weighted average value over 8 hours per day and 40 hours per week of the ACGIH (American Conference of Governmental Industrial Hygienists) in the United States;
- The MAK value (Maximale Arbeitsplatz Konzentration), 8-hour weighted average limit value, in Germany ;
- The OES (Occupational Exposure Standard) in the United Kingdom.
Occupational exposure limit values for carbon dioxide (CO2)
| On 8h | Momentary peak | IDLH(*) | |
| European Union | 5 000 ppm | – | – |
| Germany | 5 000 ppm | 10 000 ppm | – |
| United Kingdom | 5 000 ppm | 15 000 ppm – 10 min | – |
| United States | 5 000 ppm | 30 000 ppm | 40 000 ppm |
(*) Immediately dangerous to life or health
Carbon Monoxide – CO
Carbon monoxide is a colourless, odourless and toxic gas. It is the result of incomplete combustion of all types of fuel: wood, coal, oil, natural gas, etc.
Another source of carbon monoxide is human respiration. A smoker exhales up to 50 ppm.
| 0 – 3 ppm | This is a person who has not smoked at least in the last 24 hours. |
| 3 – 10 ppm |
Limit values reached in the following cases: • very little smoking |
| 10 – 20 ppm | Light smoker; these values correspond to occasional smokers, or when the last cigarette has been smoked several hours ago. |
| 21 – 50 ppm | Big to very big smoker. |
| sup. à 50 ppm | Values above 50 ppm are rarely recorded, they are observed in very heavy smokers, in those who inhale cigar or pipe smoke or smoke cannabis. |
CO prevents the body from supplying oxygen by attaching itself to the red blood cells. This causes symptoms such as headaches, malaise, nausea, vomiting, mental confusion.
Occupational exposure limit values for CO are not internationally homogeneous. The OEL of CO is generally between 20 and 50 ppm.
Occupational exposure limit values for carbon dioxide (CO)
| On 8h | Momentary peak | |
| European Union | 20 ppm | 100 ppm |
| Germany | 60 ppm | 30 ppm |
| United Kingdom | 30 ppm | 200 ppm |
| United States (OSHA) |
VLEP-8h : 50 ppm 8h-TWA : 25 ppm |
– |
| United States (NIOSH) | 35 ppm | 200 ppm |
The SUB’ROCA’s solution: the Scrub’Tech scrubber
- Carbon Dioxide (CO2) Treatment
The chemical compound in the filter cartridges produces an exothermic reaction at room temperature when in contact with CO2, according to the following chemical equation:
CO2 gas + Ca (OH)2 solid ? CaCO3 solid + H2 O liquid
- Treatment of Carbon Monoxide (CO)
The chemical compound in the filter cartridges produces an exothermic reaction at room temperature when in contact with CO, according to the following chemical equation :
2CO gas + O2 gas ? 2CO2 gas
- Air purification
A purifying process unique in the world equips the SUB’ROCA air purifier. It destroys odours, all biological pollutants (viruses, bacteria, moulds), fine particles from combustion but also chemical pollutants (VOC, formaldehyde, NOx).
- Scrub’Tech autonomy
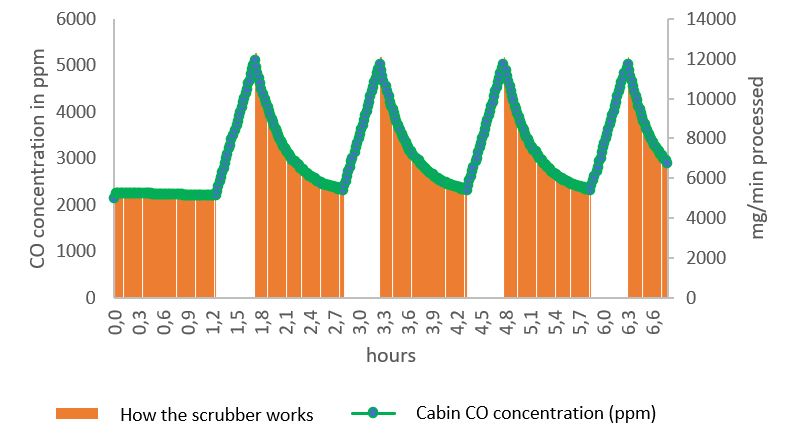
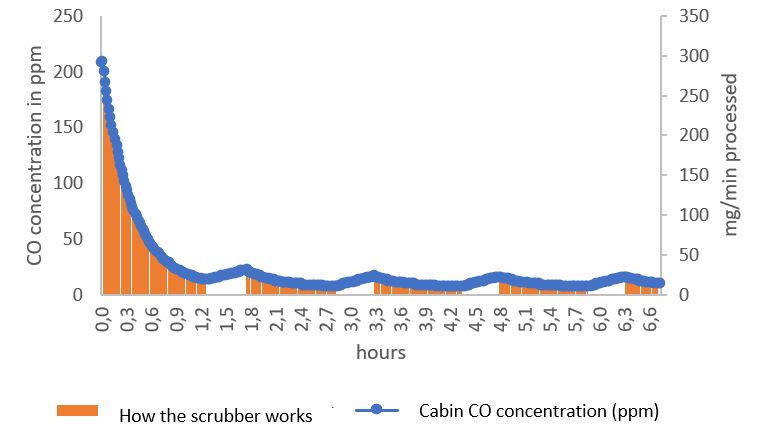
The autonomy of the Scrub’Tech was determined by SUB’ROCA’s research department thanks to the modelling of the concentration of pollutants in the air of the refuge.
The graphs below show the evolution of air quality (CO and CO2 concentration) over 6 hours in a refuge containing 24 people.
Under these conditions, the refuge’s autonomy over 24 hours is ensured by 1 CO filter cartridge and 5 CO2 filter cartridges.